Calculate The Mass In Grams Of 1.75 Moles Of H2o
Treneri
May 10, 2025 · 4 min read
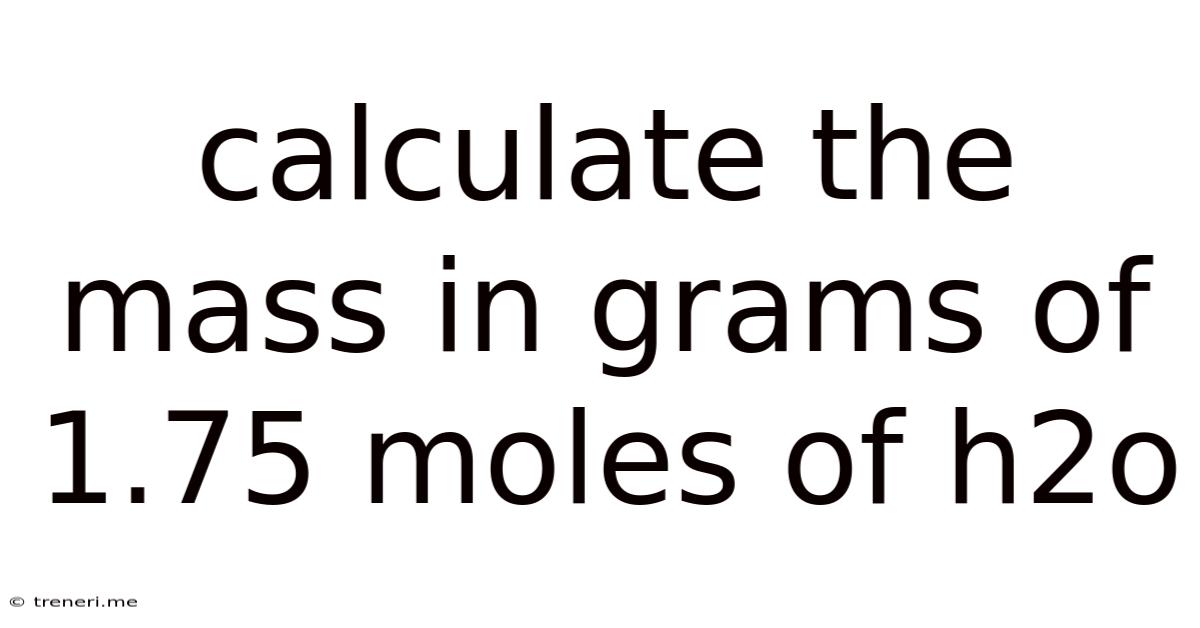
Table of Contents
Calculating the Mass of 1.75 Moles of H₂O: A Comprehensive Guide
Understanding molar mass and its application in chemistry is fundamental to various calculations. This article delves into the process of calculating the mass of 1.75 moles of water (H₂O), explaining the underlying concepts and providing a step-by-step approach. We'll also explore related concepts and practical applications to solidify your understanding.
Understanding Moles and Molar Mass
Before we jump into the calculation, let's clarify the key terms:
Mole (mol): The mole is the International System of Units (SI) base unit for the amount of substance. It's a fundamental unit in chemistry, representing a specific number of particles (atoms, molecules, ions, etc.). This number is Avogadro's number, approximately 6.022 x 10²³. Essentially, one mole of any substance contains 6.022 x 10²³ particles of that substance.
Molar Mass (g/mol): Molar mass is the mass of one mole of a substance. It's expressed in grams per mole (g/mol). To find the molar mass of a compound, you add up the atomic masses (in grams per mole) of all the atoms in its chemical formula. Atomic masses are typically found on the periodic table.
Calculating the Molar Mass of Water (H₂O)
Water, with its chemical formula H₂O, consists of two hydrogen atoms and one oxygen atom. To calculate its molar mass:
-
Find the atomic mass of hydrogen (H): The periodic table shows hydrogen's atomic mass as approximately 1.008 g/mol.
-
Find the atomic mass of oxygen (O): Oxygen's atomic mass is approximately 16.00 g/mol.
-
Calculate the molar mass of H₂O:
- Two hydrogen atoms: 2 * 1.008 g/mol = 2.016 g/mol
- One oxygen atom: 1 * 16.00 g/mol = 16.00 g/mol
- Total molar mass of H₂O: 2.016 g/mol + 16.00 g/mol = 18.016 g/mol
Calculating the Mass of 1.75 Moles of H₂O
Now that we know the molar mass of water, we can calculate the mass of 1.75 moles. The key relationship is:
Moles (mol) * Molar Mass (g/mol) = Mass (g)
-
Identify the known values:
- Moles of H₂O = 1.75 mol
- Molar mass of H₂O = 18.016 g/mol
-
Apply the formula:
- Mass of H₂O = 1.75 mol * 18.016 g/mol = 31.528 g
Therefore, the mass of 1.75 moles of H₂O is approximately 31.528 grams.
Understanding Significant Figures
In scientific calculations, it's crucial to consider significant figures. Significant figures represent the digits in a number that carry meaning contributing to its precision. The least precise measurement dictates the number of significant figures in the final answer.
In our example:
- 1.75 moles has three significant figures.
- 18.016 g/mol has five significant figures.
Since 1.75 mol has fewer significant figures, our final answer should also have three significant figures. Therefore, we round 31.528 g to 31.5 g.
Practical Applications and Further Exploration
The ability to calculate the mass of a given number of moles is essential in many chemical applications:
-
Stoichiometry: Stoichiometry involves using balanced chemical equations to determine the quantitative relationships between reactants and products in chemical reactions. Molar mass is crucial for converting between moles and grams in stoichiometric calculations.
-
Solution Preparation: Chemists frequently prepare solutions with specific concentrations. Knowing the molar mass allows them to accurately weigh out the required amount of solute to achieve the desired concentration.
-
Titrations: Titrations are analytical techniques used to determine the concentration of a solution. Calculations involving moles and molar mass are essential in titration analysis.
-
Chemical Engineering: Chemical engineers use molar mass calculations in process design, scale-up, and optimization of chemical processes.
Beyond Water: Extending the Calculation
The principle of calculating mass from moles applies to any substance. You simply need to determine the substance's molar mass using the periodic table and the chemical formula. For example, to find the mass of 2.5 moles of carbon dioxide (CO₂), you would:
-
Calculate the molar mass of CO₂: (12.01 g/mol (C) + 2 * 16.00 g/mol (O)) = 44.01 g/mol
-
Multiply the moles by the molar mass: 2.5 mol * 44.01 g/mol = 110.025 g. Rounding to the correct significant figures yields 110 g.
Troubleshooting Common Mistakes
Common mistakes when performing these calculations include:
-
Incorrect Molar Mass Calculation: Double-check your addition of atomic masses and ensure you're using the correct atomic masses from the periodic table.
-
Significant Figure Errors: Pay close attention to significant figures throughout the calculation and round your final answer appropriately.
-
Unit Errors: Always include units in your calculations and make sure they cancel correctly. If your units don't cancel to give you grams, there's an error somewhere.
Conclusion
Calculating the mass of a given number of moles is a fundamental skill in chemistry. By understanding the concepts of moles, molar mass, and significant figures, you can confidently perform these calculations for various substances. This skill is essential for various applications, ranging from simple stoichiometry problems to complex chemical engineering calculations. Remember to always double-check your work, pay attention to units and significant figures, and practice regularly to master this important concept. With consistent practice and a solid grasp of the underlying principles, you can confidently tackle more complex chemical calculations.
Latest Posts
Latest Posts
-
1 50 Cm A Pies Y Pulgadas
May 11, 2025
-
How Many Square Feet In 200 Square Meters
May 11, 2025
-
1 3 Squared In Fraction Form
May 11, 2025
-
Can I Tan In Uv 7
May 11, 2025
-
How Many Cups Of Popcorn In A Small Movie Bag
May 11, 2025
Related Post
Thank you for visiting our website which covers about Calculate The Mass In Grams Of 1.75 Moles Of H2o . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.