How Many Sig Figs In 0.2
Treneri
May 14, 2025 · 5 min read
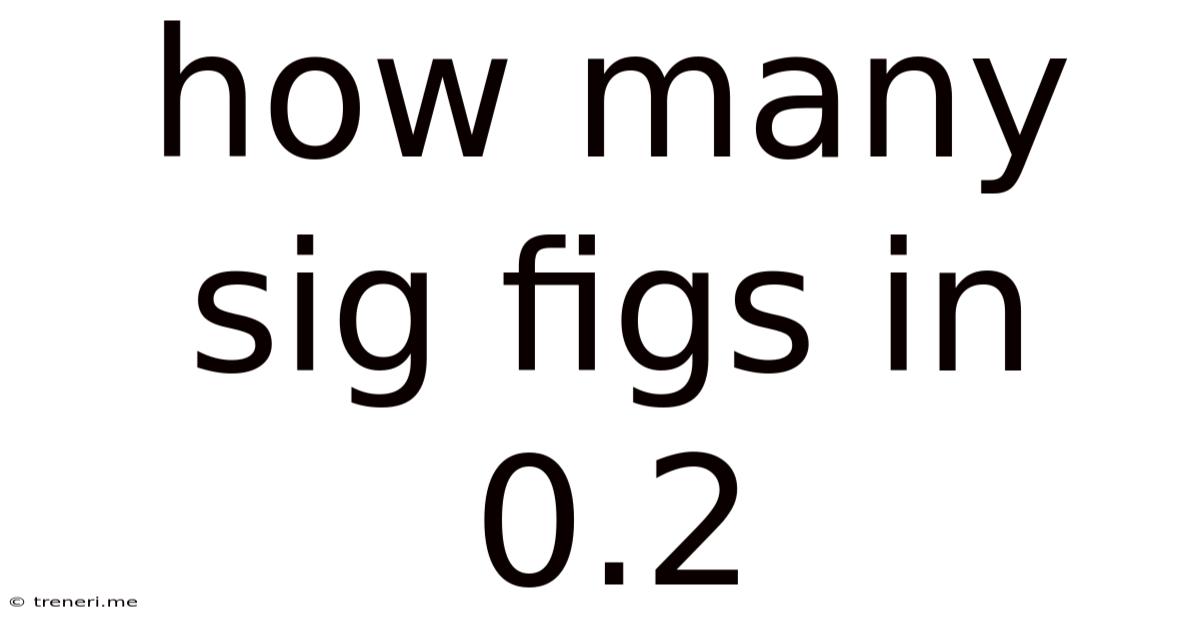
Table of Contents
How Many Significant Figures are in 0.2? A Deep Dive into Significant Figures
Determining the number of significant figures (sig figs) in a number might seem straightforward, but subtleties can arise, especially with numbers containing zeros. This article provides a comprehensive exploration of significant figures, focusing specifically on the seemingly simple number 0.2, and expanding on the broader principles to solidify your understanding.
Understanding Significant Figures
Significant figures represent the precision of a measurement. They indicate the digits in a number that carry meaning contributing to its accuracy. It's crucial in science, engineering, and any field dealing with numerical data to accurately represent the level of uncertainty associated with a measurement.
Why are significant figures important?
- Accuracy: They communicate the level of accuracy of a measurement or calculation. A measurement of 10.0 cm is more precise than a measurement of 10 cm.
- Calculations: Significant figures are used to ensure that results from calculations don't appear more precise than the original data allows. This prevents misleading conclusions.
- Scientific Communication: Consistent use of significant figures is essential for clear and reliable scientific communication.
Rules for Determining Significant Figures
Before tackling 0.2, let's review the fundamental rules for identifying significant figures:
1. Non-zero Digits: All non-zero digits are always significant. For example, in the number 245, all three digits (2, 4, and 5) are significant.
2. Zeros: Zeros can be tricky, falling into several categories:
- Leading Zeros: Zeros to the left of the first non-zero digit are not significant. They merely serve to place the decimal point. For example, in 0.0025, only the 2 and 5 are significant.
- Trailing Zeros: Trailing zeros (zeros at the end of a number) are significant only if the number contains a decimal point. For example, 1200 has two significant figures, but 1200. has four significant figures. The decimal point explicitly indicates the precision.
- Captive Zeros: Zeros between non-zero digits are always significant. For example, in 1002, all four digits are significant.
3. Scientific Notation: When a number is expressed in scientific notation (e.g., 2.5 x 10³), all digits in the coefficient are significant.
Applying the Rules to 0.2
Now, let's apply these rules to the number 0.2:
The number 0.2 has only one significant figure. The zero is a leading zero, and therefore, it is not significant. The only significant digit is the 2.
Why is the zero not significant?
The leading zero simply serves as a placeholder to position the decimal point correctly. It doesn't contribute to the precision of the measurement that 0.2 represents. It's about the actual measured value, and in this case, the measurement's precision only extends to the tenths place, represented by the digit 2.
Illustrative Examples and Contrast
Let's consider some examples to further clarify the concept and differentiate it from similar-looking numbers:
- 0.02: This number also has one significant figure (the 2). The leading zeros are not significant.
- 0.20: This number has two significant figures. The trailing zero after the decimal point indicates a greater level of precision. It implies that the measurement was made to the hundredths place, even though the value in that place is zero.
- 200: This is ambiguous. It could have one, two, or three significant figures depending on the context. Without more information, we can't definitively say. To eliminate ambiguity, scientific notation should be used (e.g., 2.0 x 10² for two significant figures, or 2.00 x 10² for three).
- 200.: This number has three significant figures due to the presence of the decimal point. The trailing zeros are now significant.
Practical Applications and Implications
The seemingly simple matter of significant figures significantly impacts various scientific and engineering calculations. For example:
- Error Propagation: Calculations involving measurements with different levels of precision (i.e., different numbers of significant figures) require careful consideration of error propagation. The result of a calculation cannot be more precise than the least precise measurement used in the calculation.
- Experimental Design: Choosing appropriate measuring instruments and techniques to achieve a desired level of precision directly influences the number of significant figures obtained in experimental data.
- Data Analysis: Understanding significant figures helps in interpreting data correctly and avoiding misinterpretations stemming from inaccurate representation of precision.
Advanced Considerations and Ambiguities
While the rules are generally straightforward, ambiguities can arise, particularly with trailing zeros in numbers without decimal points. In such cases, scientific notation is the preferred method to avoid any confusion. This convention removes ambiguity regarding the number of significant digits involved.
Ambiguity Resolution Techniques:
- Contextual Clues: In certain situations, the context of the number might offer clues about its precision. For example, if a measurement is stated as "approximately 200 meters", it suggests lower precision than if it were stated as "200.0 meters."
- Explicit Statements: Researchers sometimes explicitly state the number of significant figures involved to avoid any misunderstanding.
Conclusion: The Importance of Precision
In summary, the number 0.2 contains only one significant figure. Understanding significant figures is critical for accurate scientific communication, reliable data analysis, and appropriate error propagation in calculations. Mastering these principles ensures your work reflects the true precision of your measurements and calculations, preventing misinterpretations and enhancing the credibility of your findings. Always strive for clarity and precision in your numerical representation, and when in doubt, utilize scientific notation to eliminate ambiguity. Remember, attention to detail in significant figures reflects a commitment to scientific rigor and accuracy. This careful approach prevents errors and ensures confident communication of results. The seemingly simple number 0.2 therefore serves as a potent reminder of the importance of understanding the nuances of significant figure representation.
Latest Posts
Latest Posts
-
What Is 90 Days From April 19 2024
May 14, 2025
-
How Many Ml Is 1 4 Oz
May 14, 2025
-
How Many 2x4 In A Unit
May 14, 2025
-
Greatest Common Factor Of 25 And 90
May 14, 2025
-
What Is 50 Percent Of 12
May 14, 2025
Related Post
Thank you for visiting our website which covers about How Many Sig Figs In 0.2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.