2.5 X 10 24 Molecules To Moles
Treneri
May 14, 2025 · 5 min read
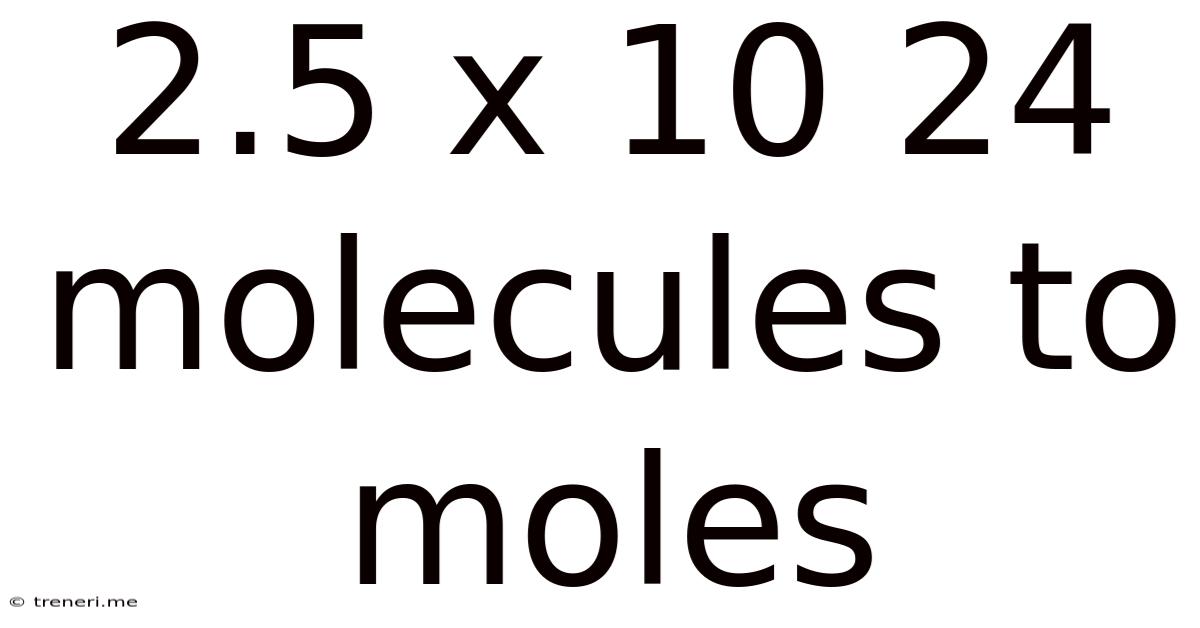
Table of Contents
2.5 x 10<sup>24</sup> Molecules to Moles: A Comprehensive Guide
Converting between molecules and moles is a fundamental concept in chemistry. Understanding this conversion is crucial for various stoichiometric calculations and mastering chemical reactions. This comprehensive guide will walk you through the process of converting 2.5 x 10<sup>24</sup> molecules to moles, explaining the underlying principles and providing practical examples. We'll also delve into the broader context of the mole concept and its significance in chemistry.
Understanding the Mole Concept
Before diving into the conversion, let's solidify our understanding of the mole. The mole (mol) is a fundamental unit in chemistry that represents a specific number of particles, be it atoms, molecules, ions, or formula units. This number is known as Avogadro's number, approximately 6.022 x 10<sup>23</sup>. It's essentially a counting unit, much like a dozen (12) or a gross (144).
One mole of any substance contains Avogadro's number of particles. This means:
- 1 mole of carbon atoms contains 6.022 x 10<sup>23</sup> carbon atoms.
- 1 mole of water molecules contains 6.022 x 10<sup>23</sup> water molecules.
- 1 mole of sodium chloride (NaCl) formula units contains 6.022 x 10<sup>23</sup> NaCl formula units.
This consistent relationship between the mole and Avogadro's number is the cornerstone of stoichiometry, allowing us to relate the amounts of reactants and products in chemical reactions.
Converting Molecules to Moles: The Formula
The conversion between molecules and moles is straightforward. We use Avogadro's number as the conversion factor. The formula is:
Moles = (Number of molecules) / (Avogadro's number)
Conversely, if you have the number of moles and want to find the number of molecules:
Number of molecules = (Moles) x (Avogadro's number)
Calculating Moles from 2.5 x 10<sup>24</sup> Molecules
Now, let's apply this formula to convert 2.5 x 10<sup>24</sup> molecules to moles.
1. Identify the given information:
We are given 2.5 x 10<sup>24</sup> molecules.
2. Apply the formula:
Moles = (2.5 x 10<sup>24</sup> molecules) / (6.022 x 10<sup>23</sup> molecules/mol)
3. Perform the calculation:
Notice that the "molecules" unit cancels out, leaving us with "moles" as the unit. The calculation yields:
Moles ≈ 4.15 moles
Therefore, 2.5 x 10<sup>24</sup> molecules is approximately equal to 4.15 moles.
Significance of the Mole in Chemical Calculations
The mole's importance extends far beyond simple conversions. It forms the basis for numerous chemical calculations, including:
1. Stoichiometry:
Stoichiometry involves calculating the quantities of reactants and products in chemical reactions. The mole provides a bridge between the macroscopic world (grams, liters) and the microscopic world (atoms, molecules). Balanced chemical equations represent the molar ratios of reactants and products. For example, in the reaction 2H<sub>2</sub> + O<sub>2</sub> → 2H<sub>2</sub>O, 2 moles of hydrogen react with 1 mole of oxygen to produce 2 moles of water.
2. Molar Mass:
The molar mass of a substance is the mass of one mole of that substance in grams. It's numerically equal to the atomic or molecular weight. This allows us to easily convert between mass (grams) and moles, a critical step in many chemical calculations. For example, the molar mass of water (H<sub>2</sub>O) is approximately 18.015 g/mol.
3. Molar Volume:
One mole of any ideal gas occupies a volume of 22.4 liters at standard temperature and pressure (STP). This relationship allows us to calculate the volume of a gas given its number of moles, or vice versa.
4. Concentration:
In solutions, concentration is often expressed in molarity (M), which represents moles of solute per liter of solution. Molarity is a crucial parameter for understanding reaction rates, equilibrium, and other solution properties.
Practical Applications and Examples
The conversion between molecules and moles is used extensively in various chemical contexts. Here are a few examples:
-
Determining the number of molecules in a given mass: If you know the mass of a substance and its molar mass, you can calculate the number of moles, and then use Avogadro's number to find the number of molecules.
-
Calculating the amount of reactant needed: In industrial processes or laboratory experiments, calculating the required amount of reactant in moles is essential for achieving the desired product yield.
-
Analyzing reaction yields: By comparing the theoretical yield (calculated from stoichiometry) to the actual yield, you can determine the percentage yield of a chemical reaction. This is crucial for evaluating reaction efficiency and optimizing processes.
-
Understanding pharmaceutical dosages: Pharmaceutical dosages are often expressed in milligrams or grams. Converting these masses to moles is critical for understanding the number of molecules of the drug being administered.
Advanced Concepts and Considerations
While the basic conversion is straightforward, some considerations might arise in more complex scenarios:
-
Dealing with impure substances: If a sample is not 100% pure, you need to account for the percentage purity when performing calculations. The actual number of molecules will be lower than calculated based on the total mass.
-
Working with mixtures: When dealing with mixtures of different substances, you might need to perform calculations for each component separately and then sum the results to get the total number of molecules.
-
Handling very large or very small numbers: Scientific notation is essential for handling extremely large or small numbers encountered in chemistry, ensuring accurate calculations and avoiding errors.
Conclusion
Converting 2.5 x 10<sup>24</sup> molecules to moles, as we've demonstrated, is a fundamental skill in chemistry. Understanding the mole concept and its relationship to Avogadro's number is crucial for mastering stoichiometry and various other chemical calculations. The ability to confidently perform these conversions is essential for anyone pursuing studies or a career in chemistry, related scientific fields, or even advanced high school chemistry. This comprehensive guide has provided a robust foundation for understanding and applying this critical concept effectively. Remember to always double-check your calculations and units to ensure accuracy. The practice of these calculations will enhance your understanding and build your confidence in tackling more complex chemical problems.
Latest Posts
Latest Posts
-
How Long Is 25 000 Minutes
May 14, 2025
-
Cost Of Shiplap Per Square Foot
May 14, 2025
-
What Is 90 Days From May 22 2024
May 14, 2025
-
How Many Days Away Is June 1st
May 14, 2025
-
Round 614 To The Nearest Ten
May 14, 2025
Related Post
Thank you for visiting our website which covers about 2.5 X 10 24 Molecules To Moles . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.