Density Is Mass Per Unit Volume
Treneri
May 11, 2025 · 6 min read
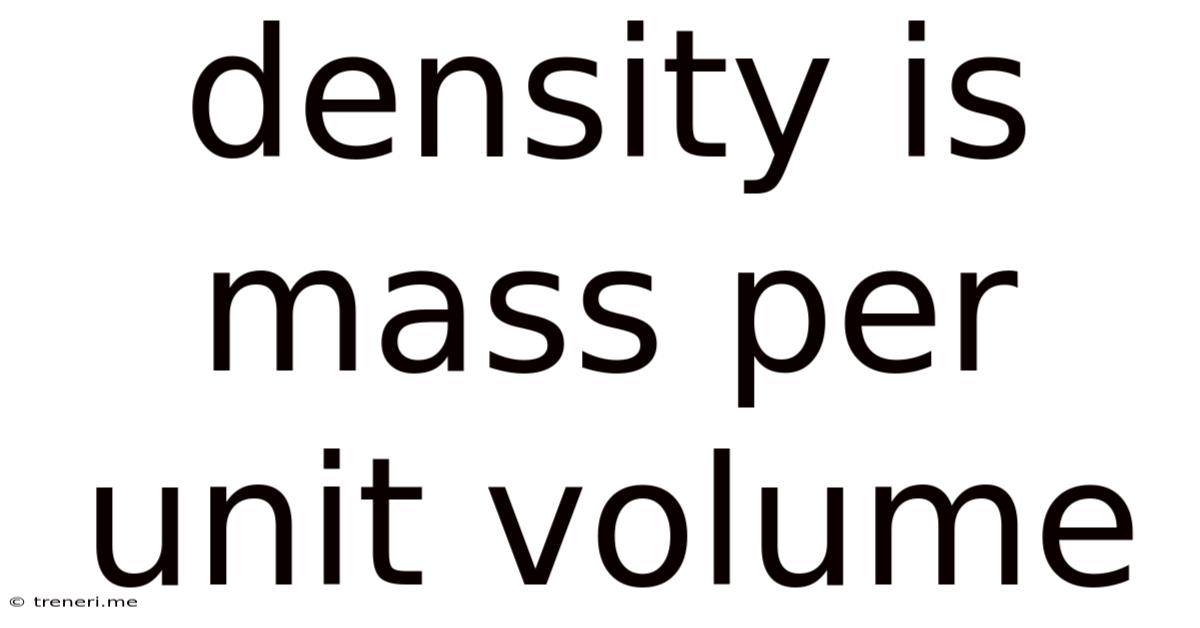
Table of Contents
Density: Mass Per Unit Volume – A Deep Dive
Density, a fundamental concept in physics and chemistry, is a measure of how much mass is packed into a given volume. Simply put, it's mass per unit volume. Understanding density is crucial in various fields, from materials science and engineering to meteorology and oceanography. This comprehensive article will explore density in detail, covering its definition, calculation, units, applications, and factors influencing it. We'll also delve into the relationship between density and other physical properties, and look at some real-world examples to solidify your understanding.
Defining Density: Mass Packed Tight
Density (ρ, pronounced "rho") is defined as the ratio of an object's mass (m) to its volume (V). Mathematically, this is expressed as:
ρ = m/V
This seemingly simple equation holds immense significance. It tells us how compact the matter within an object is. A high density means a lot of mass is squeezed into a small volume, while a low density signifies a more spread-out distribution of mass.
Units of Density
The units of density are derived from the units of mass and volume. Common units include:
- kg/m³ (kilograms per cubic meter): This is the SI unit of density.
- g/cm³ (grams per cubic centimeter): Frequently used in chemistry and materials science. Note that 1 g/cm³ = 1000 kg/m³.
- g/mL (grams per milliliter): Often used for liquids and solutions. Equivalent to g/cm³.
The choice of unit depends on the context and the scale of the measurement. For example, the density of water is approximately 1 g/cm³ or 1000 kg/m³.
Calculating Density: A Practical Approach
Calculating the density of an object requires determining both its mass and its volume. Mass is typically measured using a balance or scale. Measuring volume, however, depends on the object's state:
Measuring the Volume of Solids:
- Regularly shaped solids: For objects with well-defined geometric shapes (cubes, spheres, cylinders), the volume can be calculated using geometric formulas. For example, the volume of a cube is side³.
- Irregularly shaped solids: For irregularly shaped objects, the volume can be determined using water displacement. Submerge the object in a graduated cylinder filled with a known volume of water. The difference in water levels before and after submersion represents the object's volume.
Measuring the Volume of Liquids:
The volume of liquids is readily measured using graduated cylinders, beakers, or volumetric flasks.
Measuring the Volume of Gases:
Measuring the volume of gases is more complex and often requires specialized equipment, such as gas syringes or respirometers, taking into account temperature and pressure.
Factors Affecting Density
Several factors can influence the density of a substance:
Temperature:
Temperature significantly affects density, especially in fluids. As temperature increases, the kinetic energy of particles increases, causing them to move further apart. This leads to an increase in volume and, consequently, a decrease in density. Conversely, decreasing temperature leads to an increase in density. This is why hot air rises and cold air sinks. This temperature dependence is crucial in applications like thermometry and convection.
Pressure:
Pressure primarily affects the density of gases. Increasing pressure compresses the gas molecules closer together, reducing the volume and increasing the density. Liquids and solids are much less compressible, and their density changes relatively little with pressure changes. This is a key concept in understanding atmospheric pressure and the behavior of gases in various systems.
Composition:
The density of a mixture or solution depends on the densities and proportions of its components. For example, adding salt to water increases the solution's density because salt is denser than water. This principle is used in various separation techniques like density gradient centrifugation.
Phase:
The density of a substance typically differs significantly between its solid, liquid, and gaseous phases. Solids generally have the highest density, followed by liquids, and then gases. This is due to the differing arrangements and interactions between the particles in each phase. The notable exception is water, where ice (solid) is less dense than liquid water.
Applications of Density
Density's importance extends across diverse scientific and engineering fields:
Materials Science:
Density is a crucial parameter in materials selection. Engineers and designers consider the density of materials when designing structures, vehicles, and other products, balancing strength, weight, and cost. Materials with high strength-to-weight ratios (low density and high strength) are highly desirable in aerospace and automotive applications.
Oceanography:
Oceanographers use density measurements to understand ocean currents, stratification, and mixing processes. Differences in water density due to temperature and salinity drive these processes, shaping the ocean's circulation and influencing marine ecosystems. Density gradients play a crucial role in the vertical distribution of marine organisms.
Meteorology:
Density differences in air masses due to temperature and humidity are the driving forces behind weather patterns. Warm, less dense air rises, while cool, denser air sinks, creating pressure differences that lead to wind and precipitation. Understanding air density is fundamental to weather forecasting and climate modeling.
Medicine:
Density measurements are used in various medical applications, including bone density scans (osteoporosis diagnosis) and blood tests (measuring hematocrit, the proportion of red blood cells). These measurements provide valuable insights into overall health and potential health risks.
Density and Other Physical Properties: Interrelationships
Density is closely linked to other physical properties:
- Specific Gravity: Specific gravity is the ratio of a substance's density to the density of a reference substance, typically water. It's a dimensionless quantity.
- Buoyancy: An object floats if its density is less than the density of the fluid it's submerged in. This principle is governed by Archimedes' principle.
- Pressure: In fluids, pressure increases with depth due to the weight of the overlying fluid, which is directly related to its density.
Real-World Examples: Understanding Density in Action
Let's illustrate density's practical relevance with some examples:
- Hot air balloons: Hot air balloons rise because the heated air inside the balloon is less dense than the surrounding cooler air.
- Ice floating on water: Ice is less dense than liquid water, a crucial property for aquatic life as it prevents bodies of water from freezing solid in winter.
- Determining the purity of a substance: The density of a pure substance is a constant value at a given temperature and pressure. Deviations from the expected density can indicate impurities.
- Separating mixtures: Techniques like density gradient centrifugation separate components of a mixture based on their differing densities.
Conclusion: Mastering the Concept of Density
Density, a seemingly simple concept, underpins countless phenomena in the natural world and technological advancements. By understanding its definition, calculation, influencing factors, and applications, we gain a deeper appreciation for the fundamental role it plays in various scientific disciplines and engineering practices. This article has provided a comprehensive overview, equipping you with the knowledge to analyze and interpret density-related problems and appreciate its pervasive influence in our world. Further exploration into specific applications and related concepts will only deepen this understanding and reveal even more fascinating facets of this fundamental property of matter.
Latest Posts
Latest Posts
-
Cuanto Dura El Embarazo De Una Perra Raza Pequena
May 12, 2025
-
60 Is What Percent Of 90
May 12, 2025
-
Convert Cubic Feet To Tons Calculator
May 12, 2025
-
0 83 Rounded To The Nearest Tenth
May 12, 2025
-
Whats The Best Uv Index For Tanning
May 12, 2025
Related Post
Thank you for visiting our website which covers about Density Is Mass Per Unit Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.